Simple Definition Of Electron Energy Level
This model however is incorrect and outdated. All the electrons sharing a shell are degenerate meaning they have the same amount of energy.
 Energy Level Diagram Different Energy Shells Around The Nucleus
Energy Level Diagram Different Energy Shells Around The Nucleus
Splitting the Shells into Subshells.

Simple definition of electron energy level. The Fermi level lies between the valence band and conduction band because at absolute zero temperature the electrons are all in the lowest energy state. The region that might contain an electron is more like a cloud or atmosphere except a spherical probability only applies when an atom only has a single electron. Energy level - a definite stable energy that a physical system can have.
An electron is a very small piece of matter. An electron can possess different forms of energy- kinetic energy when it is accelerated through a potential difference potential energy when it is placed in the vicinity of a positive or negatively charged ion rotational energy because of its motion around the nucleus and around its own axis or it can gain energy from thermal fluctuations in the surrounding environment. The term shell has been replaced with the term energy levels because the term shell insinuated that electrons circled the atom in fixed orbits like planets circle the sun.
According to quantum theory only certain energy levels are possible. Its symbol is e. Energy levels electron shells shells 1 The possible locations around an atom where electrons having specific energy values quantum number may be found.
Energy levels inside an atom are the specific energies that electrons can have when occupying specific orbitals. Orbitals and the Atomic Nucleus. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus.
It was discovered by J. Electrons can be excited to higher energy levels by. The term energy level is most commonly used in reference to the electron configuration in atoms or molecules.
An electrons lowest energy level is like the fundamental frequency of a vibrating string while higher energy levels are like harmonics. The orbits have quantized sizes and energies. The electron is a subatomic particle.
Used especially of the state of electrons in atoms or molecules. Shown here is the first Balmer transition in which an electron jumps from orbit n 3 to orbit n 2 producing a photon of red light with an energy of 189 eV and a wavelength of 656 nanometres. Every atom is made of one or more electrons that surround the nucleus of the atom.
As with classical potentials the. Simply defined as the different states of potential energy for electrons in an atom. The higher the shell the higher the energy of its electron s.
In other words the energy spectrum can be quantized. Because the binding energy of a Rydberg electron is proportional to 1 r and hence falls off like 1 n2 the energy level spacing falls off like 1 n3 leading to ever more closely spaced levels converging on the first ionization energy. Ilektrn enrj levl atomic physics A quantum-mechanical concept for energy levels of electrons about the nucleus.
These closely spaced Rydberg states form what is commonly referred to. Shells around the nucleus are occupied by electrons Each shell n is labeled as a number and is numbered 1 2 3 4 etc. Energy Levels and the Atomic Model.
The highest energy level that an electron can occupy at the absolute zero temperature is known as the Fermi Level. An electron can also be separate from any atom. Electron energies are functions of each particular atomic species.
A quantum mechanical system can only be in certain states so that only certain energy levels are possible. The first element in a period of the periodic table introduces a new principal energy level. It is believed to be an elementary particle because it cannot be broken down into anything smaller.
In chemistry the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atoms nucleus. Each shell is actually an energy level. One of the stable states of constant energy that may be assumed by a physical system used especially of the quantum states of electrons in atoms and of nuclei called also energy state Examples of energy level in a Sentence Recent Examples on the Web An energy level will be back that was clearly missing last year.
This level is denoted by the principal quantum number n.
 Atomic Spectrum Definition Absorption Emission Study Com Physics Topics Lesson Energy Level
Atomic Spectrum Definition Absorption Emission Study Com Physics Topics Lesson Energy Level
 Energy Level Definition Equation W Diagrams
Energy Level Definition Equation W Diagrams
 Pin By Carly Lindstrom On Inspirations For Pendants Bohr Model Atom Model Atom
Pin By Carly Lindstrom On Inspirations For Pendants Bohr Model Atom Model Atom
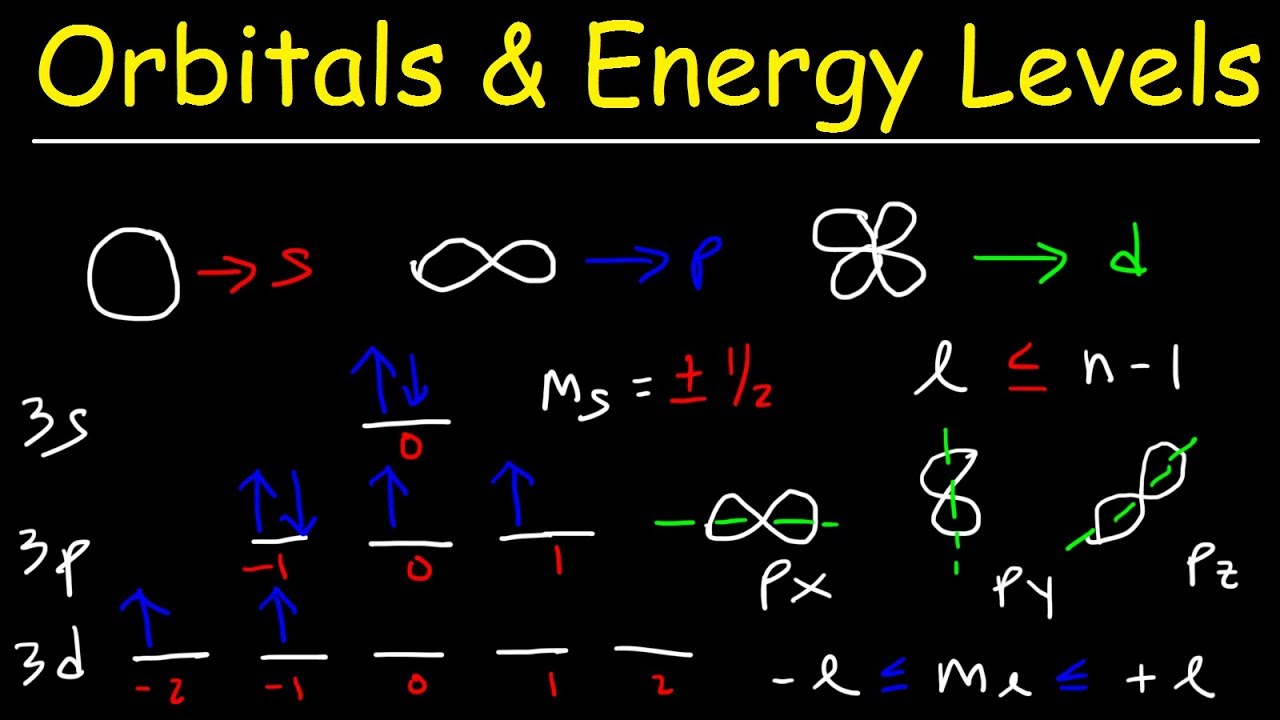 Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube
Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube
 Valence Electron Chemical Bond Science Student Science Facts
Valence Electron Chemical Bond Science Student Science Facts
 Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube
Orbitals Atomic Energy Levels Sublevels Explained Basic Introduction To Quantum Numbers Youtube
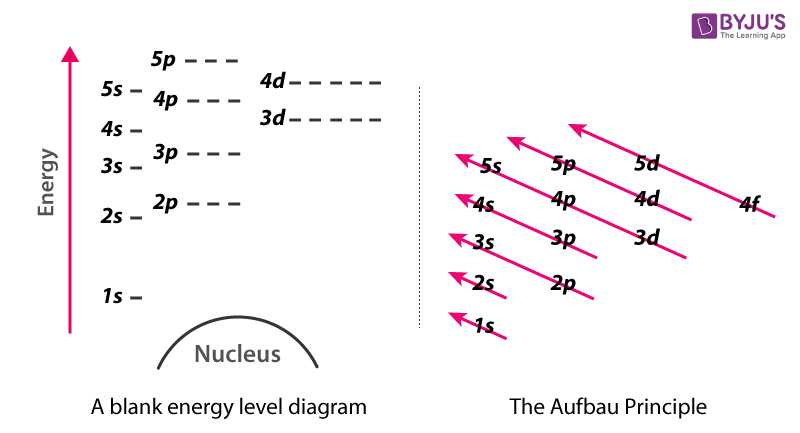 Energy Level Diagram Different Energy Shells Around The Nucleus
Energy Level Diagram Different Energy Shells Around The Nucleus
 Valence Electrons Are Electrons On The Outermost Shell Of An Atom Electrons 8th Grade Science Earth Science
Valence Electrons Are Electrons On The Outermost Shell Of An Atom Electrons 8th Grade Science Earth Science
 Electron Affinity Google Search Electron Affinity Chemistry Periodic Table
Electron Affinity Google Search Electron Affinity Chemistry Periodic Table
 Atom Orbits And Energy Levels Britannica
Atom Orbits And Energy Levels Britannica
 Quantum Numbers Energy Level Chemistry Quantum
Quantum Numbers Energy Level Chemistry Quantum
 Card Sort Activity Electron Configuration Electron Configuration Sorting Cards Free Science Worksheets
Card Sort Activity Electron Configuration Electron Configuration Sorting Cards Free Science Worksheets
 How To Find The Number Of Valence Electrons Using A Periodic Table Electrons Energy Level Energy
How To Find The Number Of Valence Electrons Using A Periodic Table Electrons Energy Level Energy
 Atomic Energy Levels Video Khan Academy
Atomic Energy Levels Video Khan Academy
 Valence Electrons The Electrons In The Outer Most Electron Shell Are Called Valence Electrons The Shell Co Reading Instruction Physical Science Science Nerd
Valence Electrons The Electrons In The Outer Most Electron Shell Are Called Valence Electrons The Shell Co Reading Instruction Physical Science Science Nerd
 The Octet Rule Of Electron Configuration Surfguppy Chemistry Made Easy Visual Learning Octet Rule Electron Configuration Chemistry
The Octet Rule Of Electron Configuration Surfguppy Chemistry Made Easy Visual Learning Octet Rule Electron Configuration Chemistry
 Atom Animated Gif Atomic Structure Structure Definition Simple Machines
Atom Animated Gif Atomic Structure Structure Definition Simple Machines

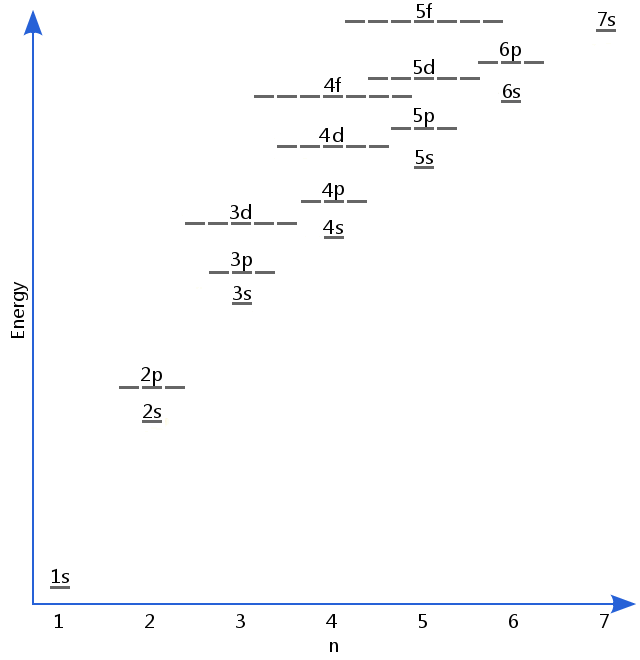
Post a Comment for "Simple Definition Of Electron Energy Level"